New industry evidence demands RWD disruption in pharma
Evidence for an RWD overhaul in pharma
Reuters Events, in partnership with Prognos Health, surveyed 215 brand, marketing, commercial strategy, medical affairs, and data science professionals in the pharmaceutical industry to understand current challenges and opportunities with real-world data (RWD) for therapy commercialization.
Your peers revealed an RWD status quo fraught with challenges and a paradigm ripe for disruption. Here are some of the most telling results:
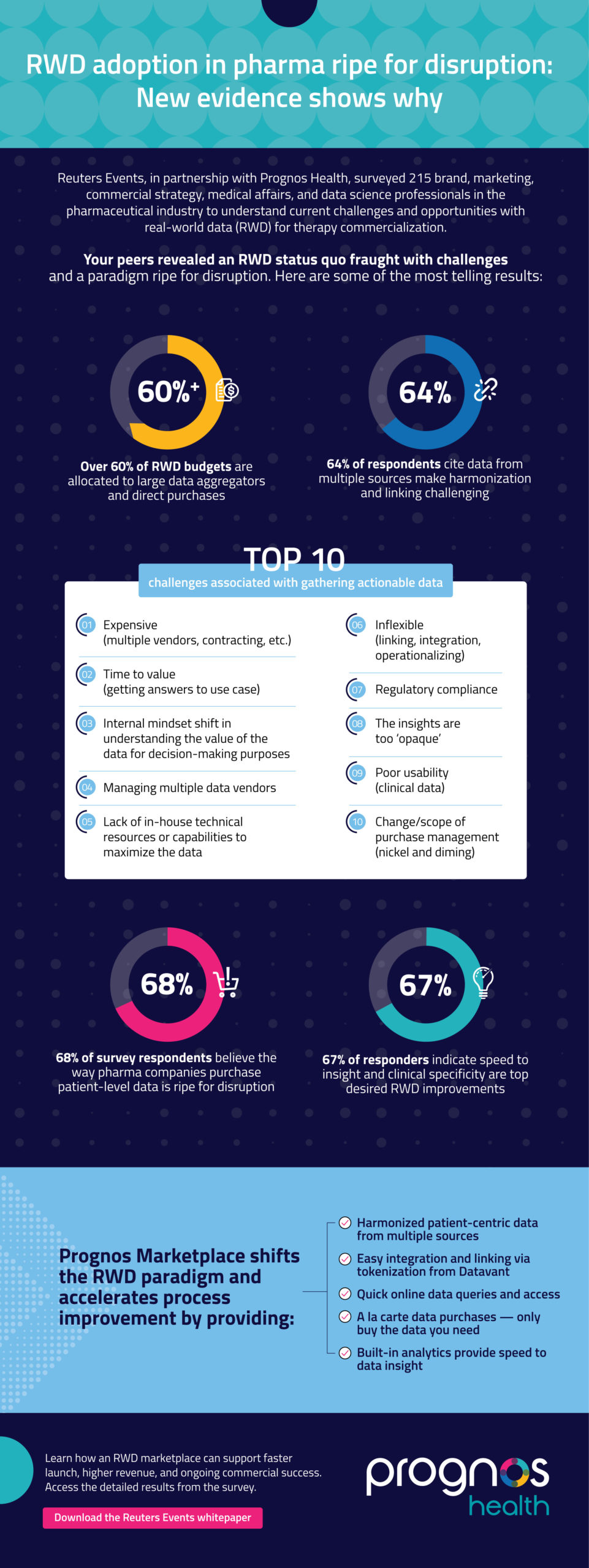
Over 60% of RWD budgets are allocated to large data aggregators and direct purchases
Top 10 challenges associated with gathering actionable data
- Expensive (multiple vendors, contracting, etc.)
- Time to value (getting answers to use case)
- Internal mindset shift in understanding the value of the data for decision-making purposes
- Managing multiple data vendors
- Lack of in-house technical resources or capabilities to maximize the data
- Inflexible (linking, integration, operationalizing)
- Regulatory compliance
- The insights are too ‘opaque’
- Poor usability (clinical data)
- Change/scope of purchase management (nickel and diming)
- Double buying (across aggregators)
64% of respondents cite data from multiple sources make harmonization and linking challenging
68% of survey respondents believe the way pharma companies purchase patient-level data is ripe for disruption
67% of responders indicate speed to insight and clinical specificity are top desired RWD improvements
Prognos Marketplace shifts the RWD paradigm and accelerates process improvement by providing:
- Harmonized patient-centric data from multiple sources
- Easy integration and linking via tokenization from Datavant
- Quick online data queries and access
- A la carte data purchases — only buy the data you need
- Built-in analytics provide speed to data insight
Learn how an RWD marketplace can support faster launch, higher revenue, and ongoing commercial success. Access the detailed results from the survey.
Download the Reuters Events whitepaper